Mammalian cell lines are being increasingly used for the manufacture of a range of biological medicines. Unfortunately, mammalian cells are vulnerable to genetic variation and contamination if indefinitely maintained by serial passage. Fortunately, they can be cryopreserved and stored in a stable condition at ultra-low temperatures until required.
The standardised preparation of cell lines is important to ensure the quality and safety of biological product during commercial production. The first key step in this process is the establishment of a Master Cell Bank (MCB) and Working Cell Bank (WCB).
Master Cell Bank (MCB)
An MCB is defined as an aliquot of a single pool of cells that generally has been prepared from the selected cell clone under defined conditions, dispensed into multiple containers, and stored under defined conditions. The MCB is used to derive all working cell banks (WCB). The testing performed on a new MCB (from a previous initial cell clone, MCB, or WCB) should be the same as for the MCB unless justified.
Working Cell Bank (WCB)
The WCB is prepared from aliquots of a homogeneous suspension of cells obtained from culturing the MCB under defined culture conditions.
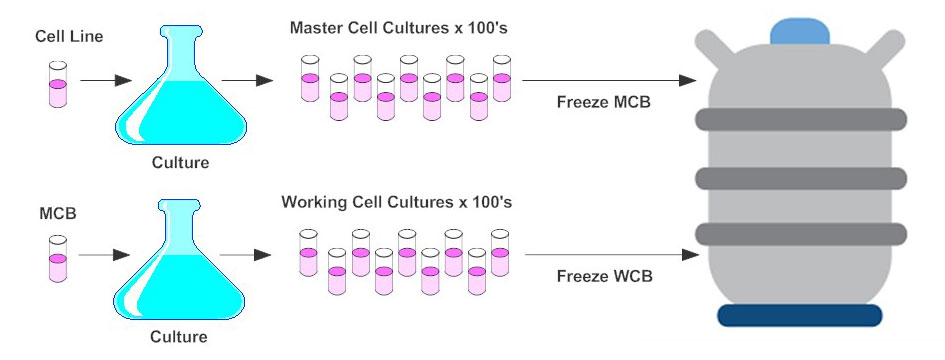
The Master Cell Bank (MCB) is derived and expanded from a one-parent cell culture (See figure). The MCB is produced using standardised laboratory procedures and characterized depending on the strain to ensure proper identity and demonstrate the cell bank is free from accidental contamination. The Working Cell Bank (WCB) is prepared from one or more MCB vials and tested in similarly to the MCB before being used in production.
If prepared correctly and properly managed a Master/Working Cell Bank can provide reproducible and dependable supplies of identical cultures over many decades
Biostór offer cGMP-compliant MCB & WCB storage services to clients who wish to maintain backup copies of their valuable cell lines.
Guidance Documents
| ICH Topic Q5D (MAR 1998) – Quality of Biotechnological Products: Derivation and Characterisation of Cell Substrates Used for Production of Biotechnological/Biological Products |
| FDA Guidance for Industry (FEB 2010) -Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications |
| ICH Q5B Guideline for Industry (FEB 1996)- Quality of Biotechnological Products: Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products |
| WHO Technical Report Series, No.978, Annex 3 – Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicinal products and for the characterization of cell banks |
| Directive 2009/41/EC – European Directive on the contained use of Genetically Modified Micro-organisms (GMMs) |
| GMO (Contained Use) Regulations 2001 to 2010. |
